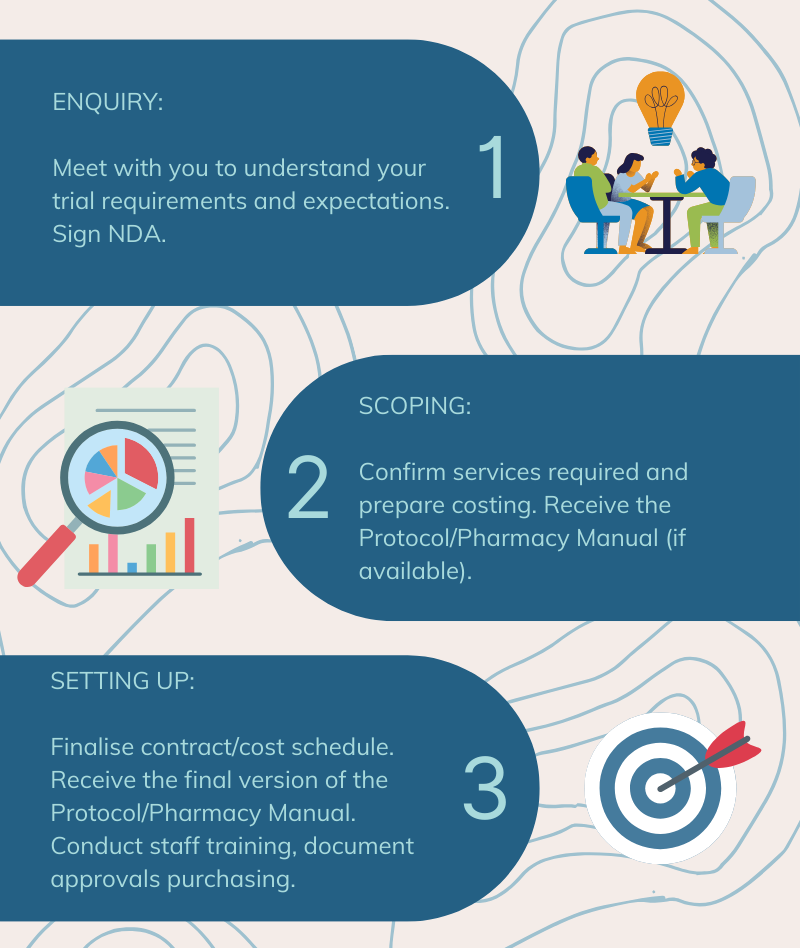
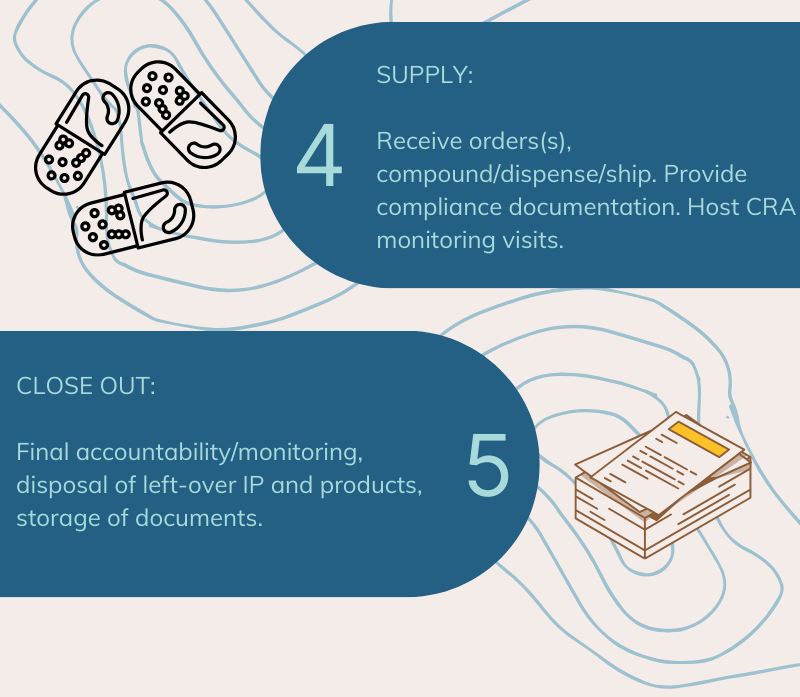
CompoundLabs Has Been Providing Support For Clinical Trials Since 2016
We are experienced in small scale GP practice clinical trials to larger multi-centre sponsor led trials and have collaborated with hospitals, universities, and specialized clinical research facilities in the successful execution of their studies.
Our GCP accredited pharmacists understand the needs of the study sponsor(s) and/or site and can customize processes to meet the study's requirements.
We work closely with the study sponsors, study investigators, site personnel and CRAs throughout the study process (from before commencement of the study until close-out).
This ensures a smooth and on-time running of the trial, including ensuring all deliveries of IPs are on time and within the specifications (documentation and temperature) required.
CompoundLabs has stringent processes and comprehensive documentation in its day-to-day operations, with enhanced procedures for clinical trials. We have built in additional processes beyond the minimum requirements to facilitate the smooth and straightforward monitoring by CRAs.
We can help with a range of services for your study that include (but are not limited to) the following:
- Procurement (including IP, capsules, fillers, packaging etc.)
- Preparation of IP and matching placebo products (non-sterile capsules, creams, powders, liquids, and troches/lozenges)
- Preparation of 'disguised' commercially available tablet/capsule and a matching placebo capsule
- Randomization plan and emergency break envelopes
- Storage at ambient, refrigerated (4°C to 8°C) or freezer (-25°C to 0°C) conditions
- Controlled drugs storage at ambient and refrigerated conditions
- Labelling (blinded/unblinded) as per protocol/Medsafe requirements
- Dispensing (individual participant dispensing is required under current legislation)
- Return, accountability and destruction (including controlled drugs) of any unused products
- Storage of all records for 10 years (or longer if required by the sponsor/site)
- Nationwide delivery under controlled temperatures with traceability
- Local hand delivery/pick up options available
At CompoundLabs, we understand the timing of dosing is crucial in clinical research. Therefore, we prioritise the compounding, dispensing and delivery of the medicines to sites, ensuring that dosing schedules are met.
Visit Us
We take pride in our compounding facilities, which is why we offer clinical research clients the opportunity to take a tour of our premises.
If you would like to visit our facility or discuss how we could help you with your clinical trial compounding needs, please contact us to organise an appropriate time.

Quality Is Key For CompoundLabs
We pride ourselves in always delivering optimal personalised healthcare by providing high quality medications and advice.
Have Questions? Contact Us:
(09) 442 1727
P.O. Box 101-142
North Shore Mail Centre 0742
Auckland
New Zealand
62C Diana Drive
Wairau Valley
Auckland 0627
Please let us know your query below and we'll get back to you as soon as possible. Our opening hours are 8:30am - 5:00pm Monday to Friday.